New IDSA/AASLD Guidelines for Hepatitis C John Scott, MD, MScAssociate Professor, UW SoMAsst Director, Liver Clinic, Harborview Medical Center Presentation prepared by Maggie Shuhart, MD and John Scott, MD Last UpdatedTreatmentNaive Genotype 1 Four highly potent DAA combination regimens are recommended for patients with genotype 1 infection, although there are differences in the recommended regimens based on the HCV subtype, the presence or absence of baseline NS5A resistanceassociated substitutions (RASs), and the presence or absence of compensatedGhany MG, Strader DB, Thomas DL, Seeff LB;

Hepatitis C Guidance 19 Update American Association For The Study Of Liver Diseases Infectious Diseases Society Of America Recommendations For Testing Managing And Treating Hepatitis C Virus Infection Ghany
Aasld practice guidelines chronic hepatitis b
Aasld practice guidelines chronic hepatitis b-An update on treatment of genotype 1 chronic hepatitis C virus infection 11 practice guideline by the American Association for the Study of Liver Diseases Hepatology 11 Oct 54(4)Funding for the hepatitis C guidance project is provided exclusively by AASLD and IDSA The hepatitis C guidance panel members serve as uncompensated volunteers
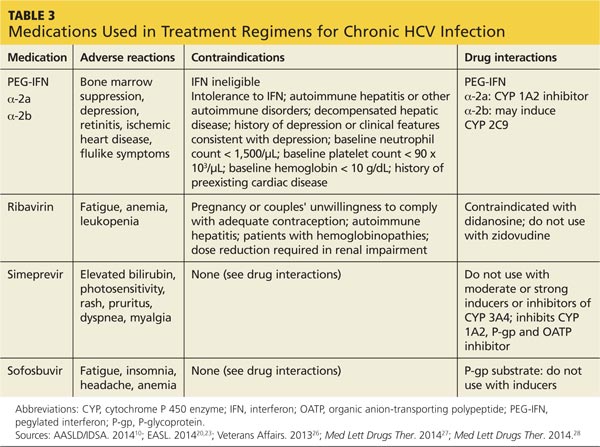



Chronic Hepatitis C Infection Bane Of Baby Boomers Clinician Reviews
To more fully characterize the quality of evidence supporting recommendations, the Practice Guidelines Committee of the AASLD requires a Class (reflecting benefit versus risk) and Level (assessing strength or certainty) of Evidence to be assigned and reported with each recommendation (Table 1, adapted from the American College of Cardiology and the AmericanFrom the Clinical Liver Disease journal Diagnosis and Management of Autoimmune Hepatitis in Adults and Children A Patient‐Friendly Summary of the 19 AASLD Guidelines Hepatitis B The Hepatitis B Foundation is a national nonprofit organization dedicated to finding a cure and improving the quality of life for those affected by hepatitis BHepatitis C guidance AASLDIDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus / AASLD/IDSA HCV Guidance Panel In Hepatology, Vol 62, No 3, , p Research output Contribution to journal › Article › peerreview
Burnout in the Pandemic Name It, Frame It and Tame It Emerging Topic Conference Chronic Hepatitis B From the Population to New Molecules and BackInstructions In the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America (AASLDIDSA) Guidance titled Recommendations for Testing, Managing, and Treating Hepatitis C, a rating system is utilized for the level of evidence and the strength of the recommendation In the Methods section, see Table 2– held by the American Association for the Study of Liver Diseases – found that universal screening of pregnant women at risk for hepatitis C virus
Diseases AASLD and Infectious Diseases Society of America IDSA) joined forces to develop guidance for the management of hepatitis C in this rapidly moving field The International Antiviral SocietyUSA, which has experience in developing treatment guidelines in HIV disease, was invited to join the effort as a collaboNORTHWEST AIDS EDUCATION AND TRAINING CENTER New IDSA/AASLD Guidelines for Hepatitis C John Scott, MD, MSc Associate Professor, UW SoM Asst Director, Liver Clinic, Harborview Medical Center Presentation prepared by Maggie Shuhart, MD and John Scott, MD Last UpdatedThe American Association for the Study of Liver Diseases (AASLD) collaborated with the Infectious Diseases Society of America (IDSA) and the International Antiviral SocietyUSA to update these hepatitis C virus (HCV) guidelines The guidelines writing panel consisted of 26 specialists in hepatology and infectious disease, as well as a patient




Executive Summary Of The 18 Kdigo Hepatitis C In Ckd Guideline Welcoming Advances In Evaluation And Management Kidney International




Hepatitis C New Guidelines 14 By sld Idsa Sofosbuvir
Hepatitis C enduring material is now available Crediting claiming for this enduring material is available from through Questions?Recognizing the importance of timely guidance regarding the rapidly evolving field of hepatitis C management, the American Association for the Study of Liver Diseases (AASLD) and the Infectious Diseases Society of America (IDSA) developed a webbased process for the expeditious formulation and dissemination of evidencebased recommendationsWebinar AASLDALEH COVID19 and Liver Disease in the Americas – 21 and Beyond Webinar Mental Health &




Optimal Management Of Pediatric Hepatitis C Infection A Review Phmt
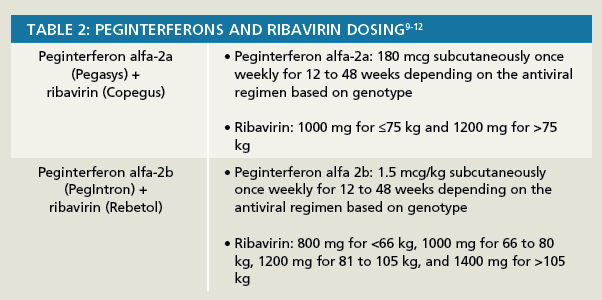



Lxryeu4ix5h1 M
Specific recommendations are based on relevant published information In an attempt to characterize the quality of evidence supporting recommendations, the Practice Guidelines Committee of the AASLD requires a category to be assigned and reported with each recommendation (Table 1) Introduction The hepatitis C virus (HCV) is a major public healthLessons HCV Epidemiology in the United States;AASLD/IDSA HCV guidance panel Recommendations for testing, managing, and treating hepatitis C Updated Internet cited




Summary Of sld Idsa And Easl Guidelines For Management Of Download Table
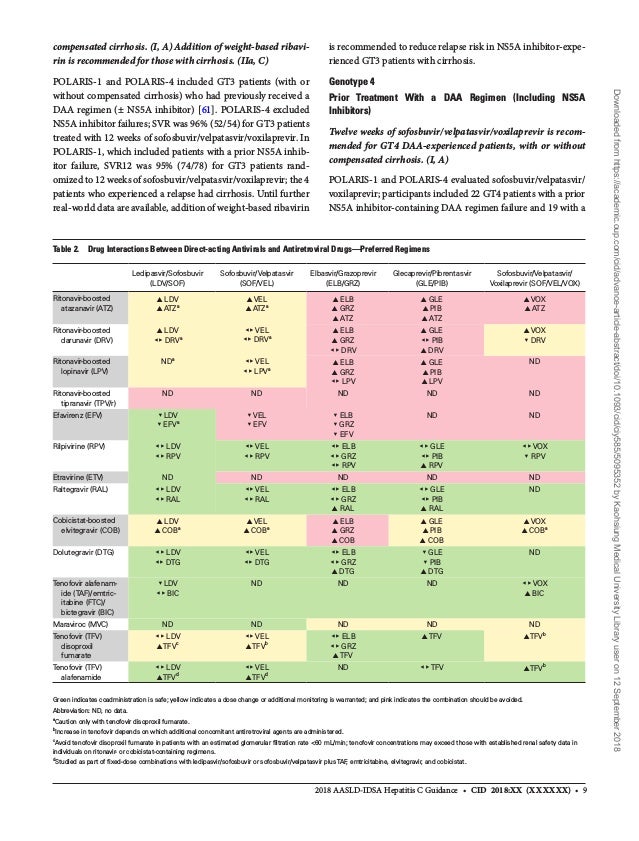



Hepatitis C Guidance 18 Update sld Idsa Recommendations For Testi
This AASLD 18 Hepatitis B Guidance is intended to complement the AASLD 16 Practice Guidelines for Treatment of Chronic Hepatitis B(1) and update the previous hepatitis B virus (HBV) guidelines from 09 The 18 updated guidance on chronic hepatitis B (CHB) includes (1) updates on treatment since the 16 HBV guidelines (notably theRECOMMENDED RATING Parents should be informed that hepatitis C is not transmitted by casual contact and, as such, children with HCV infection do not pose a risk to other children and can participate in school, sports, and athletic activities, and engage in all other regular childhood activities without restrictionsHCV Guidance Updates Recommendations for Identification and Management of Chronic Hep C Media Contacts Nola Gruneisen, AASLD, 571‐292‐3068 Lauren Martin, IDSA, () HCVguidelinesorg — a website developed by the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America to provide uptodate guidance on the management of hepatitis C




Global Hepatitis C Elimination An Investment Framework The Lancet Gastroenterology Hepatology



Quest Diagnostics Managing Hep C At Every Stage
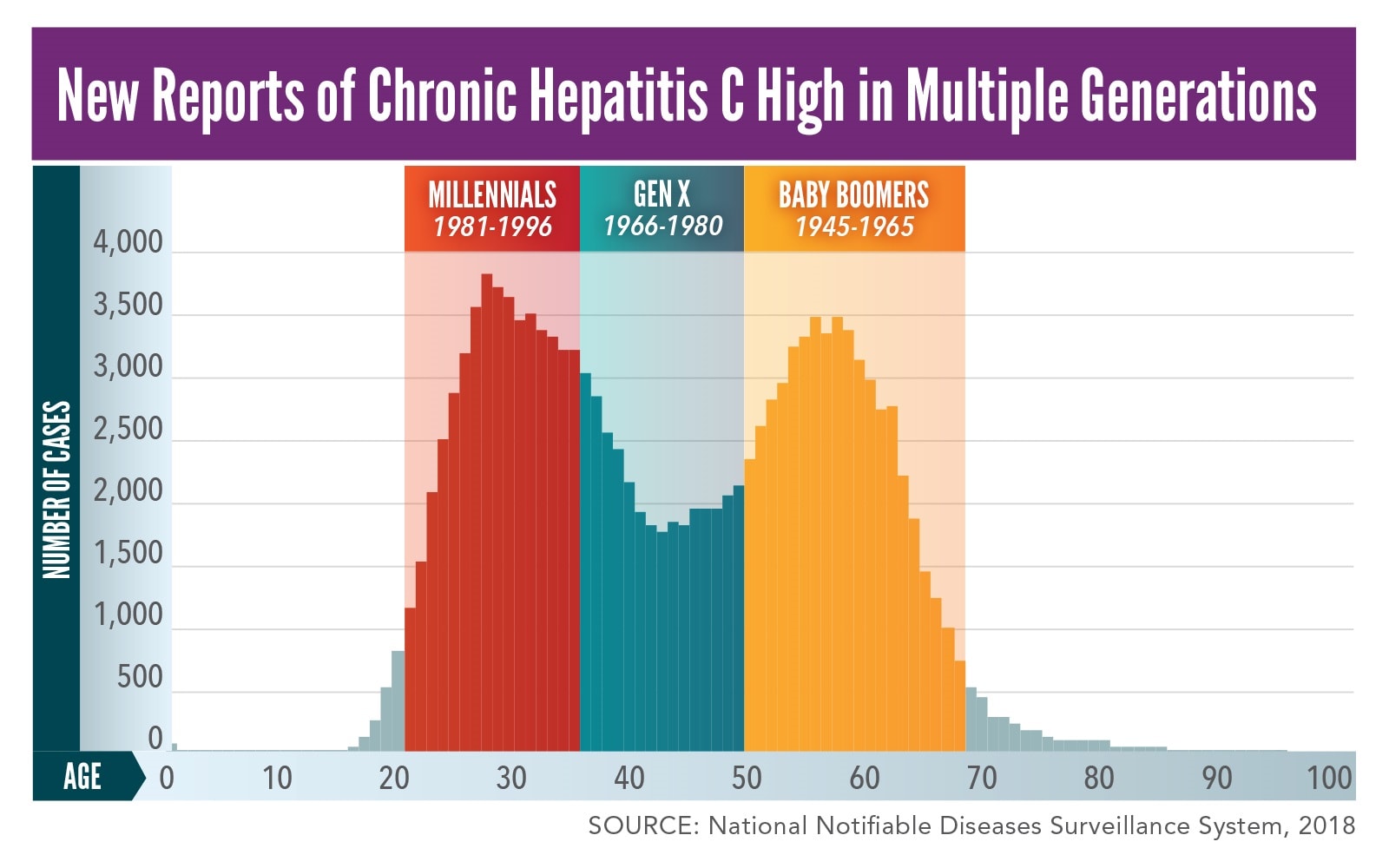
By testing and diagnosing all baby boomers with chronic HCV infection, we can get many people into care and cured, averting at least 1,000 deaths — DHHS National Viral Hepatitis Action Plan, 17 CDC, USPSTF, and AASLD/IDSA Hepatitis C Virus Screening Recommendations An individual meeting at least 1 of the criteria should receive either 1time screening or annualWard chairs the AASLD Task Force for Hepatitis Elimination Over a 13year tenure, Dr Ward directed the US CDC Division of Viral Hepatitis including hepatitis surveillance, prevention, and research At the national level, Dr Ward developed recommendations for hepatitis A and hepatitis B vaccination, hepatitis B and hepatitis C screening, andSelfStudy Module 3rd Edition CNE/CME Available Track your progress and receive CE credit Screening and Diagnosis SelfStudy CNE/CME;




Successful Hepatitis C Treatment Reduces Risk Of Liver Cancer And Death But Most Remain Untreated Aidsmap



Hepatitis C Screening Changes Treatment Advances Mdedge Family Medicine
The goal of the hepatitis C guidance is to provide uptodate recommendations for HCV care practitioners on the optimal screening, management, and treatment for adults with HCV infection in the United States, using a rigorous review process toThe HCV guidance was developed and is updated by a volunteer panel (representing the AASLD and the IDSA) of hepatology and infectious diseases clinicians with hepatitis C expertise using an evidencebased review of available data, including information presented at scientific conferences and published in peerreviewed journalsRecommendations for Hepatitis C Screening




Pdf Recent Update Of The 17 Korean Association For The Study Of The Liver Kasl Treatment Guidelines Of Chronic Hepatitis C Comparison Of Guidelines From Other Continents 17 sld Idsa And 16 Easl



Flyer Hepatitis C Screening And Treatment In People With Hiv Aids Education And Training Centers National Coordinating Resource Center Aetc Ncrc
1 Treatment considerations and choice of regimen for hepatitis C virus (HCV)infected patients a Please refer to AASLD guidelines for recommended treatment regimens and durations 2 Identifying treatment candidates a Treatment is recommended for all patients with chronic HCV infection,Hepatitis C Guidance 19 Update American Association for the Study of Liver DiseasesInfectious Diseases Society of America Recommendations for Testing, Managing, and Treating Hepatitis C Virus Infection Marc G Ghany 1 , Timothy R Morgan 2 , AASLDIDSA Hepatitis CHepatitis C Guidance 19 Update American Association for the Study of Liver Diseases–Infectious Diseases Society of America Recommendations for Testing, Managing, and Treating Hepatitis C Virus Infection Hepatitis C guidance panel members and authors and their affiliations are listed at the end of the article




Chronic Hepatitis C Infection Bane Of Baby Boomers Clinician Reviews




Summary Of sld Idsa And Easl Guidelines For Management Of Download Table
Abstract Recognizing the importance of timely guidance regarding the rapidly evolving field of hepatitis C management, the American Association for the Study of Liver Diseases (AASLD) and the Infectious Diseases Society of America (IDSA) developed a webbased process for the expeditious formulation and dissemination of evidencebased recommendationsAmerican Association for the Study of Liver Diseases Hepatology 09;The Hepatitis C GUIDELINES Pocketcard™ is endorsed by the American Association for the Study of Liver Diseases (AASLD) and is based on the latest AASLD guidelines This practical quickreference tool contains screening, diagnostics, treatment algorithms, drug therapy, dosing information, patient monitoring, and counseling points



sld Guidelines Hepatitis C 19



The Evolving Management Of Hepatitis C Virus
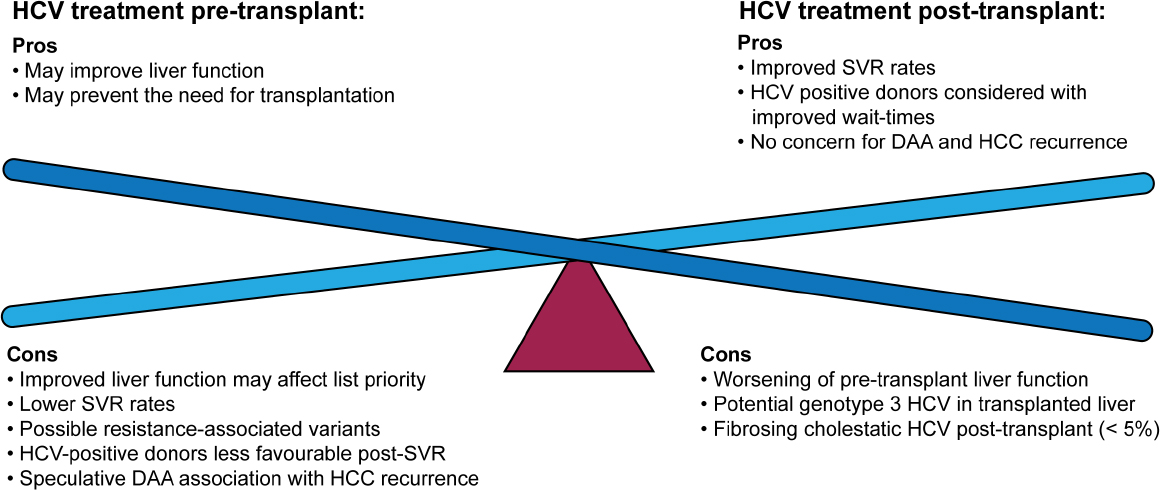
Option, recommendations reflect the best possible management for a given patient and a given point of disease progression Recommendations are graded with regard to the level of the evidence and strength of the recommendation The AASLD/IDSA/IAS–USA hepatitis C Guidance is supported by the membershipMedia Contact Nola Gruneisen Phone 571‐292‐3068 Onsite phone Email media@aasldorg (link sends email) SAN FRANCISCO – Preliminary data from a new study presented this week at The Liver Meeting®The following pages include guidance for management of patients with HCV in unique and key populations Patients With HIV/HCV Coinfection Patients With Decompensated Cirrhosis Patients Who Develop Recurrent HCV Infection Post Liver Transplantation Treatment of HCVUninfected Transplant Recipients Receiving Organs From HCVViremic Donors



English World Gastroenterology Organisation



1
The American Association for the Study of Liver Diseases, along with other societies, has updated guidelines for the treatment of hepatitis C viral infection (HCV), including the use ofAASLD Guidelines for Treatment of Chronic Hepatitis B Norah A Terrault,1 Natalie H Bzowej,2 KyongMi Chang,3 Jessica P Hwang,4 Maureen M Jonas,5 and M Hassan Murad6 See Editorial on Page 31 Objectives and Guiding Principles Guiding Principles This document presents official recommendations of the American Association for the Study ofHepatitis C guidance 18 update sldidsa recommendations for testing, managing, and treating Hepatitis C Virus infection




Hepatitis C Cancer Therapy Advisor




Hepatitis C In Pregnancy A Unique Opportunity To Improve The Hepatitis C Cascade Of Care Kushner 19 Hepatology Communications Wiley Online Library
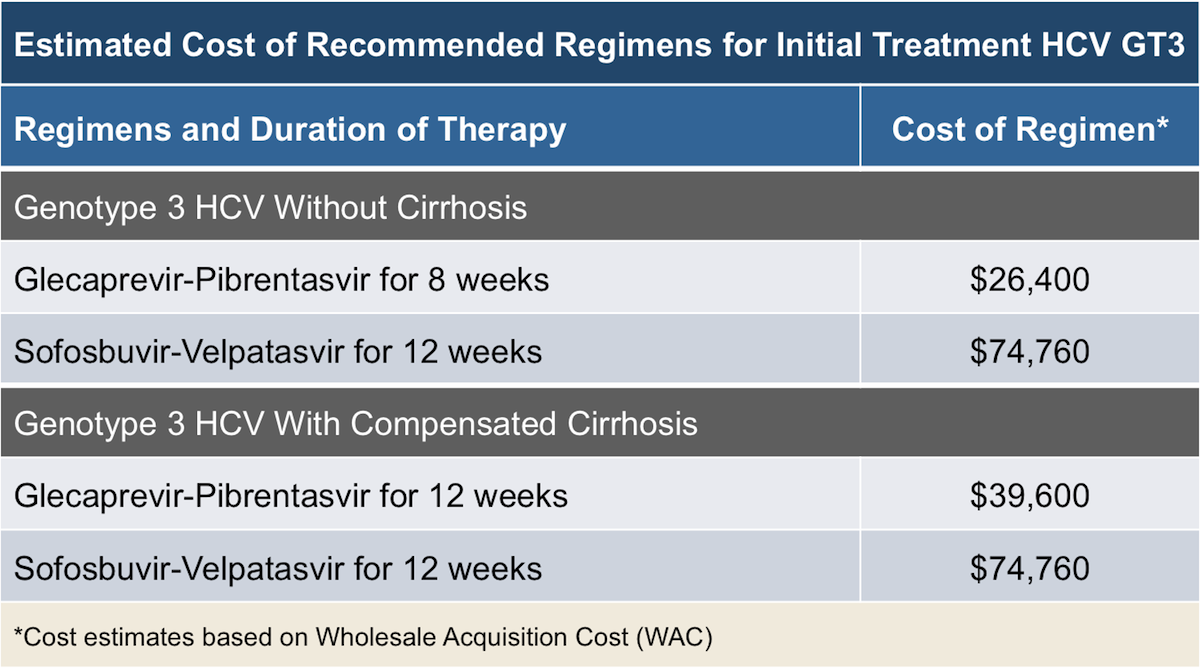
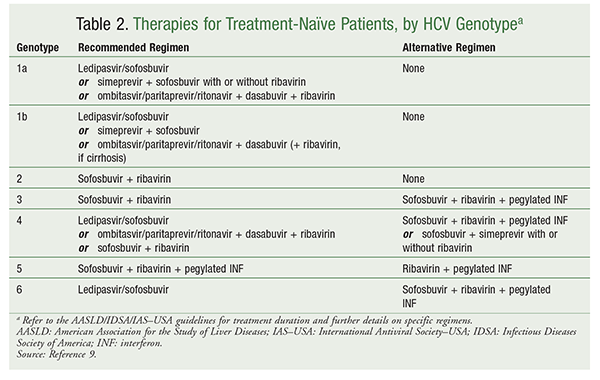
TreatmentNaive Genotype 3 The following pages include guidance for management of treatmentnaive patients with genotype 3 infection TreatmentNaive Genotype 3 Without Cirrhosis TreatmentNaive Genotype 3 With Compensated Cirrhosis Simplified HCV Treatment for TreatmentNaive Adults Without Cirrhosis Last updateThe AASLD/IDSA Guideline for Treatment of Hepatitis C Virus An InDepth Guide Testing Specifics Initial testing for HCV involves a test for the presence or absence of antiHCV antibodies, followed Treatment Efficacy With hepatitis C, the efficacy of treatments are measured in terms of rates ofThis section provides guidance on the retreatment of persons with chronic HCV infection in whom prior therapy failed The level of the evidence available to inform the best regimen for each patient and the strength of the recommendation vary and are rated accordingly (see Methods Table 2)In addition, specific recommendations are given when treatment differs for a particular group (eg,




Diagnosis And Management Of Hepatitis C American Family Physician




Recommendations For Testing Managing And Treating Hepatitis C Hcv Guidance
Recognizing the importance of timely guidance regarding the rapidly evolving field of hepatitis C management, the American Association for the Study of Liver Diseases (AASLD) and the Infectious Diseases Society of America (IDSA) developed a webbased process for the expeditious formulation and dissemination of evidencebased recommendations Launched in 14, the hepatitis C virusIn general, HBV has a significantly stronger oncogenic potential than HCV In 18, the AASLD issued the document Update on Prevention, Diagnosis, and Treatment of Chronic Hepatitis B AASLD 18 Hepatitis B Guidance This document includes recommendations for HCC surveillance in persons with chronic HBV infection1 To see the most recent recommendations pertaining to surveillance for hepatocellular carcinoma, open the document AASLD Guidelines for the Treatment of Hepatocellular Carcinoma 2 Review pages , including the recommendations at




Hepatitis C Guidance 19 Update American Association For The Study Of Liver Diseases Infectious Diseases Society Of America Recommendations For Testing Managing And Treating Hepatitis C Virus Infection Ghany




Nvhr Applauds New sld And Idsa Guidelines For Hepatitis C Screening And Treatment For At Risk Populations Hep
Recommendations reflect the best possible management for a given patient and a given point of disease progression Recommendations are rated with regard to the level of the evidence and strength of the recommendation The AASLD/IDSA Guidance on Hepatitis C is supported by the membershipbasedTable 1 Summary of the Process and Methods for the Guidance Development Table 2 Rating System Used to Rate Level of Evidence and Strength of Recommendation Table 3 Commonly Used Abbreviations and Their Expansions References Testing, Evaluation, and Monitoring of Hepatitis C Browse Topics Testing, Evaluation, and Monitoring of Hepatitis CHepatitis C Guidance 19 Update AASLD‐IDSA Recommendations for Testing, Managing, and Treating Hepatitis C Virus Infection November 18 AASLDIDSA Recommendations for Testing, Managing, and Treating Hepatitis C Virus Infection Published in Clinical Infectious Diseases Hepatitis C



2



Hepatitis C And Chronic Kidney Disease Overview Of Evaluation And Management National Kidney Foundation
AASLD/IDSA Guidelines Recommendations for Testing, Managing, and Treating Hepatitis C Jay R Kostman, MD Clinical Professor of Medicine Associate Director, Center for Viral Hepatitis Perelman School of Medicine Learning Objectives Upon completion of this presentation, learnersAASLD PRACTICE GUIDELINES Diagnosis, Management, and Treatment of Hepatitis C An Update Marc G Ghany,1 Doris B Strader,2 David L Thomas,3 and Leonard B Seeff4 This document has been approved by the AASLD, the Intended for use by physicians, these recommenda Infectious Diseases




Hepatitis C Guidance 19 Update American Association For The Study Of Liver Diseases Infectious Diseases Society Of America Recommendations For Testing Managing And Treating Hepatitis C Virus Infection Ghany




Hepatitis C Virus Infection In Patients With Cancer Impact On Clinical Trial Enrollment Selection Of Therapy And Prognosis Gastroenterology
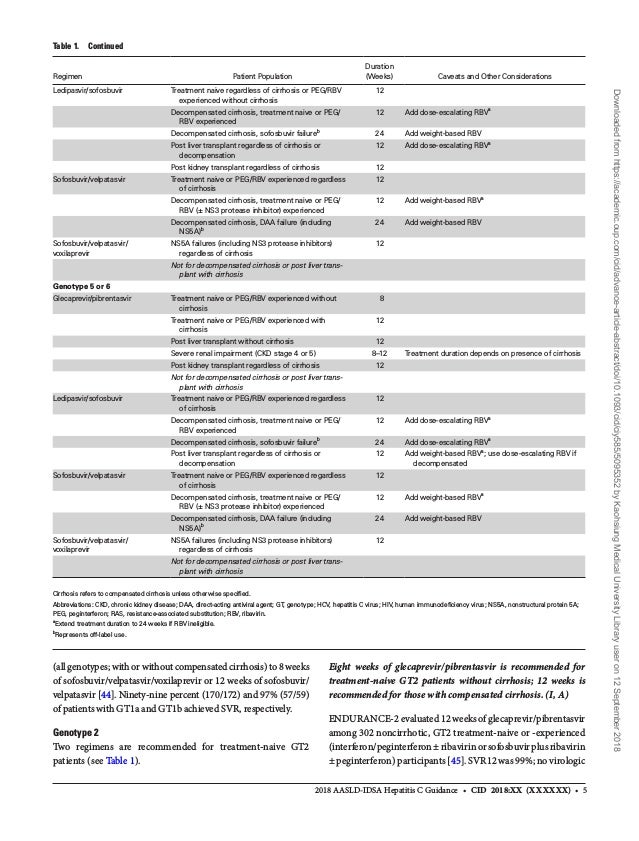



Hepatitis C Guidance 18 Update sld Idsa Recommendations For Testi




Executive Summary Of The 18 Kdigo Hepatitis C In Ckd Guideline Welcoming Advances In Evaluation And Management Kidney International




sld Recommendations For Treatment Of Chronic Hepatitis C Download Table




Table 1 From Hepatitis C Virus A Review Of Treatment Guidelines Cost Effectiveness And Access To Therapy Semantic Scholar



1
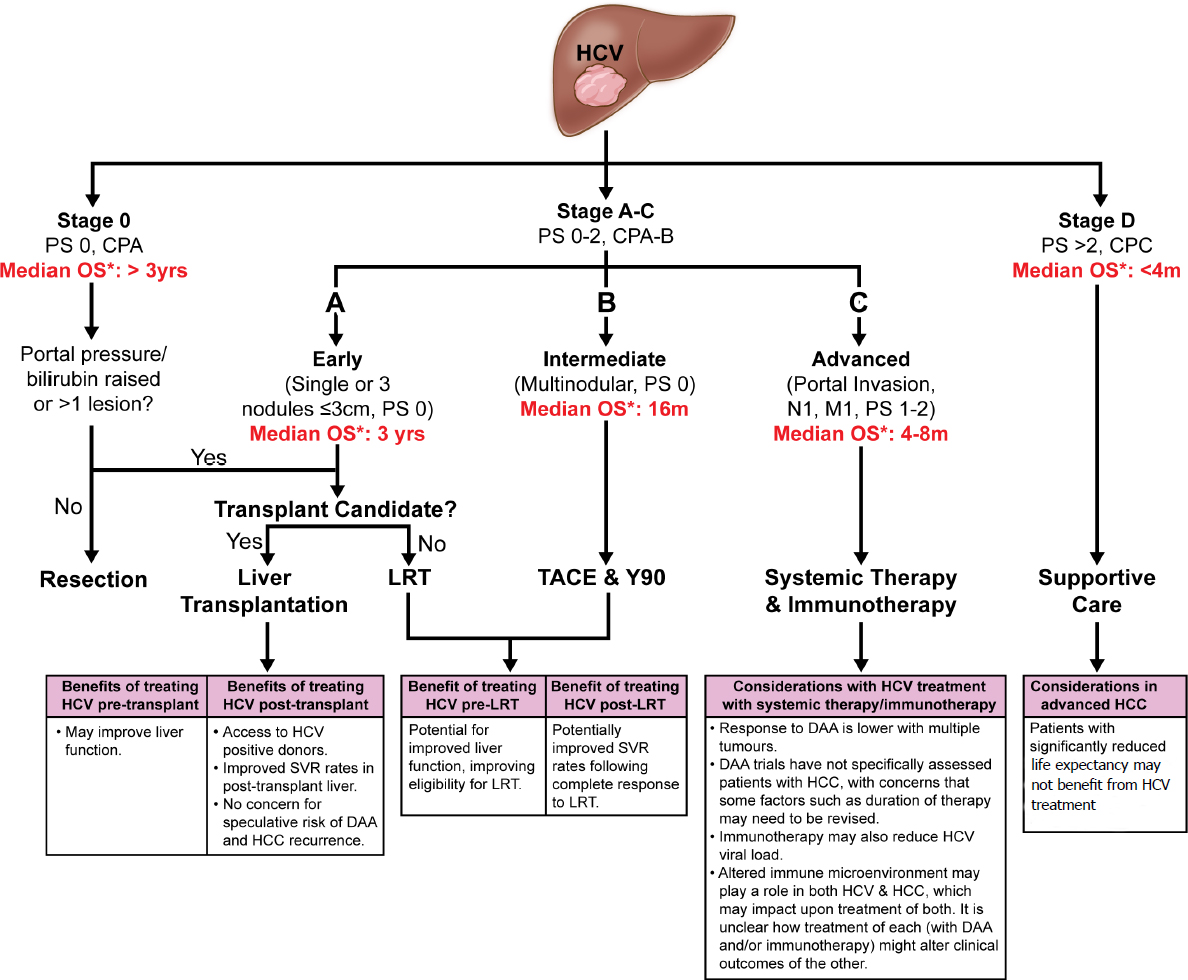



Management Of Concomitant Hepatocellular Carcinoma And Chronic Hepatitis C A Review
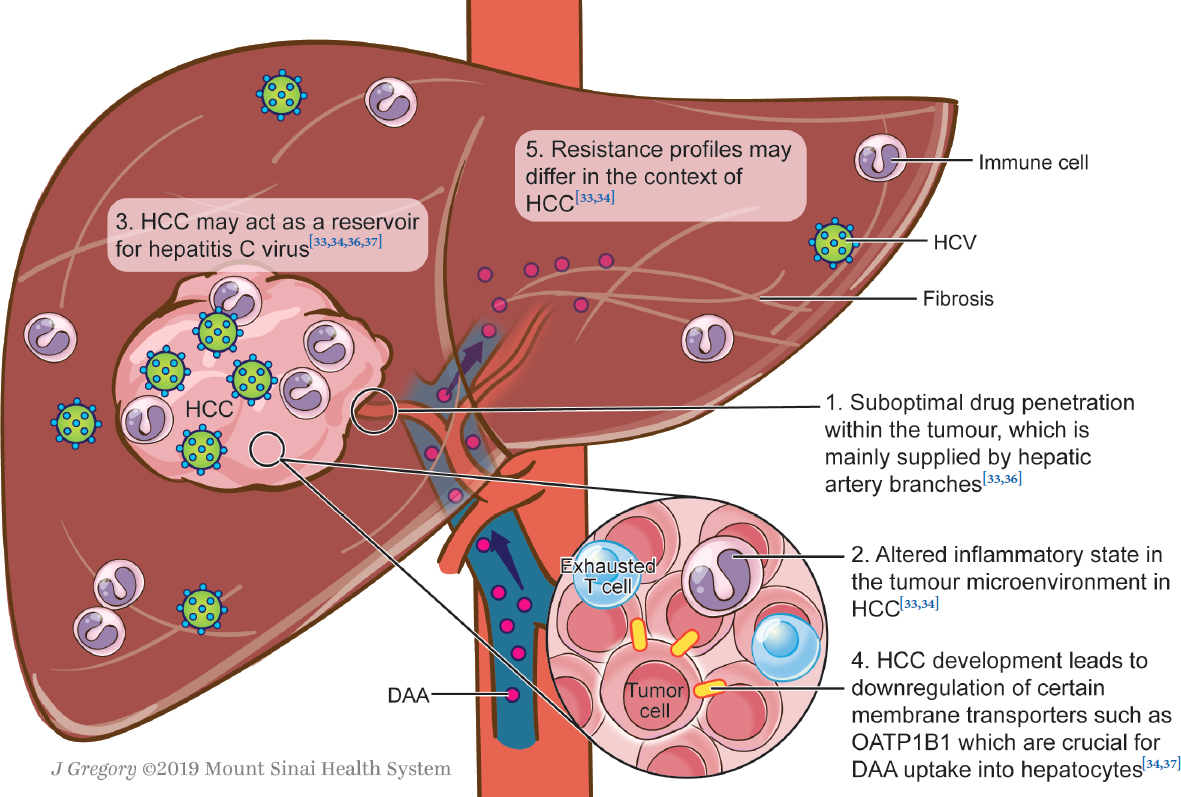



Management Of Concomitant Hepatocellular Carcinoma And Chronic Hepatitis C A Review
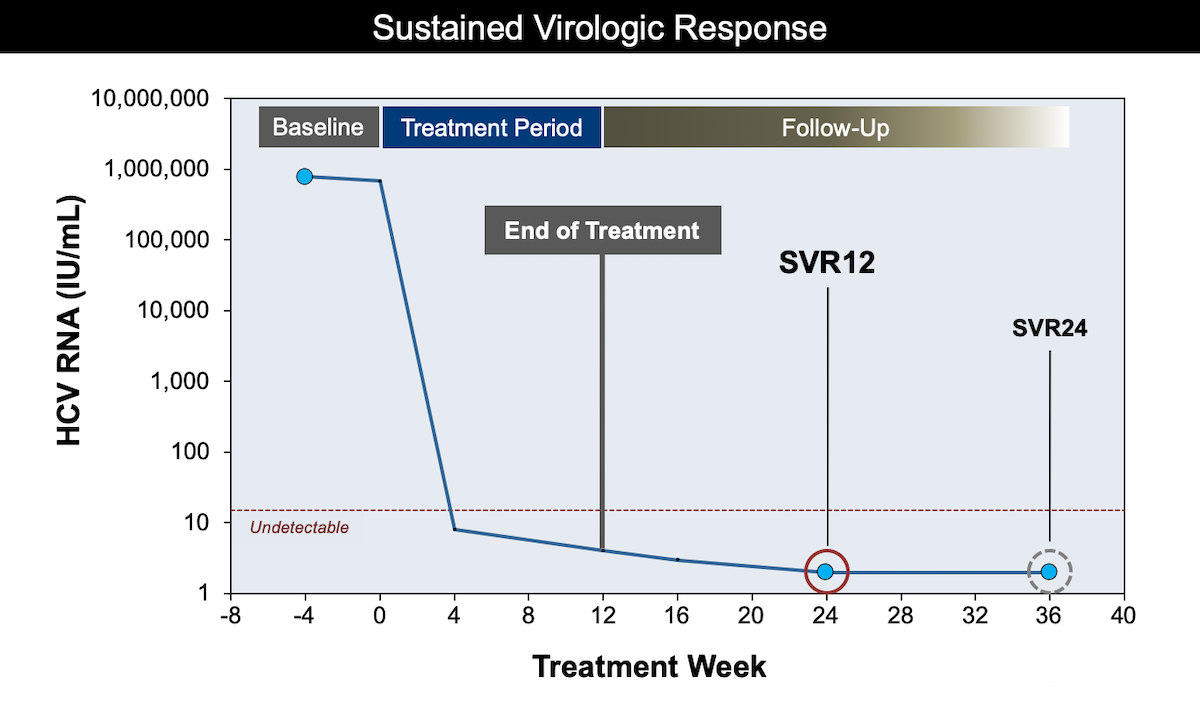



Core Concepts Monitoring During And After Hcv Treatment Treatment Of Chronic Hepatitis C Infection Hepatitis C Online




Comparison Of The Current International Guidelines On The Management Of Hcc Jhep Reports




Challenges And Perspectives Of Direct Antivirals For The Treatment Of Hepatitis C Virus Infection Journal Of Hepatology




Hepatitis C Guidance 19 Update American Association For The Study Of Liver Diseases Infectious Diseases Society Of America Recommendations For Testing Managing And Treating Hepatitis C Virus Infection Ghany



Management Of Concomitant Hepatocellular Carcinoma And Chronic Hepatitis C A Review




sld Guidelines Hepatitis C



Summary From sld 15 For Hepatitis C




Diagnosis And Management Of Hepatitis C American Family Physician



Cdc Vital Signs Dramatic Increases In Hepatitis C Cdc




Hepatitis C Global Epidemiology And Strategies For Control Clinical Microbiology And Infection



1




Hepatitis C Guidance sld Idsa Recommendations For Testing Managing And Treating Adults Infected With Hepatitis C Virus 15 Hepatology Wiley Online Library




Recommendations For Testing Managing And Treating Hepatitis C Hcv Guidance



Q Tbn And9gcqhyhq9mtwa9pa2pff0iu749gj2abuphyls Kdeijqipsez7a Usqp Cau




Diagnosis And Management Of Hepatitis C American Family Physician
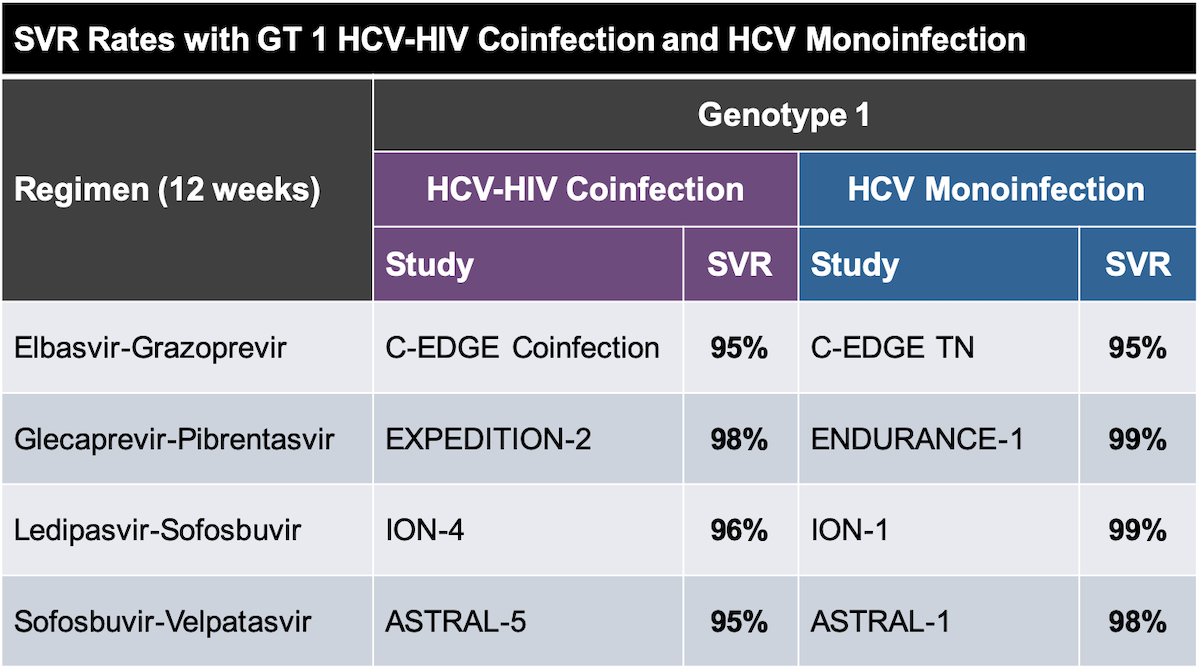



Core Concepts Treatment Of Hcv In Persons With Hiv Coinfection Treatment Of Key Populations And Unique Situations Hepatitis C Online
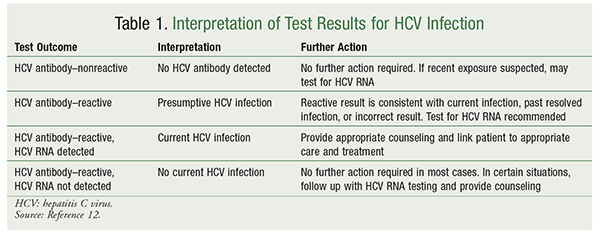



The Evolving Management Of Hepatitis C Virus




Hepatitis C And Kidney Disease A Narrative Review Sciencedirect




Comprehensive 18 sld Guidance For Chronic Hepatitis B Gutsandgrowth



Www sld Org Sites Default Files 19 06 Hbvguidance Terrault Et Al 18 Hepatology Pdf




Optimal Management Of Pediatric Hepatitis C Infection A Review Phmt




Hcv Guidance May 24 18b




Pdf Hepatitis C Guidance 18 Update sld Idsa Recommendations For Testing Managing And Treating Hepatitis C Virus Infection



Search Yield sld American Association For The Study Of Liver Download Scientific Diagram




Genotype Specific Rx




sld Guidelines
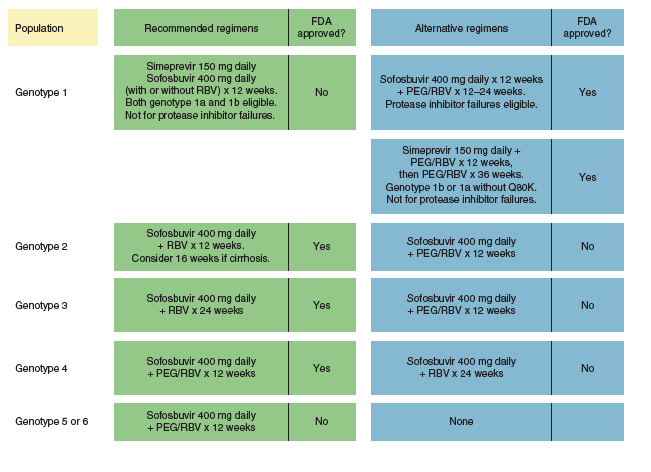



The Rapid Evolution Of Treatment Strategies For Hepatitis C Current sld Idsa Treatment Guidelines



Having Trouble Reading This Email View It In Your Browser January 14 Contents New Us Hepatitis C Treatment Guidelines New European Hepatitis C Treatment Guidelines Sofosbuvir Approval In European Union Sofosbuvir Ledipasvir Results Daclatasvir
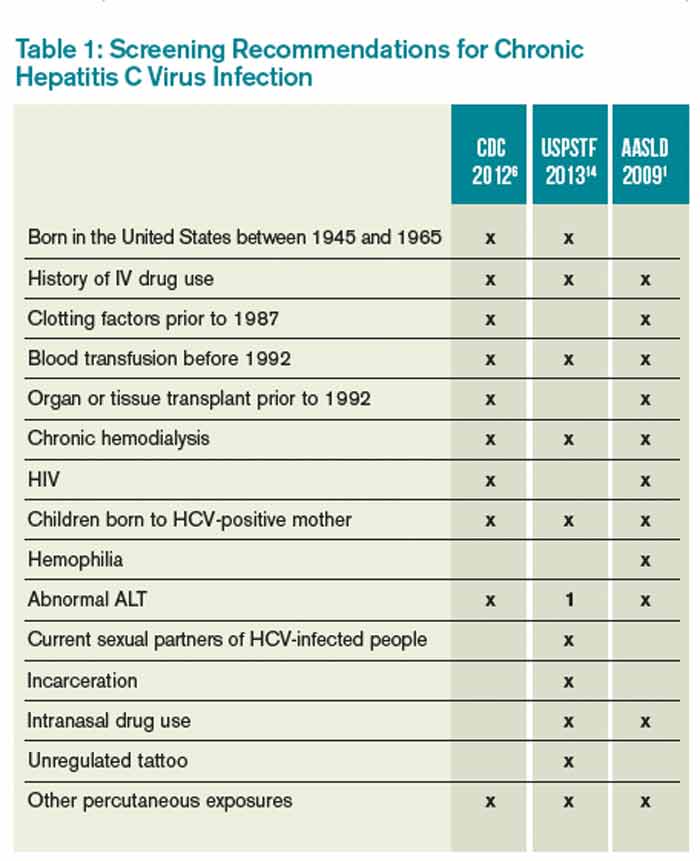



Which Patients Should Be Screened For Hepatitis C Virus Infection The Hospitalist




Hepatitis C The Lancet



Plos One Management Of Chronic Hepatitis C At A Primary Health Clinic In The High Burden Context Of Karachi Pakistan



Www Ccah Alliance Org Providerspdfs Hcv Checklist Pdf




Hcv Guidance Updates Recommendations For Identification And Management Of Chronic Hep C sld




Practice Guidelines sld




Hepatitis C Guidance 19 Update American Association For The Study Of Liver Diseases Infectious Diseases Society Of America Recommendations For Testing Managing And Treating Hepatitis C Virus Infection Ghany




Hepatitis C Laboratory Tests Wikidoc



2




Pdf Hepatitis C Guidance 18 Update sld Idsa Recommendations For Testing Managing And Treating Hepatitis C Virus Infection




sld Hbv Guidelines 18 Pdf




Kdoqi Us Commentary On The 18 Kdigo Clinical Practice Guideline For The Prevention Diagnosis Evaluation And Treatment Of Hepatitis C American Journal Of Kidney Diseases




Hepatitis C Guidance 19 Update American Association For The Study Of Liver Diseases Infectious Diseases Society Of America Recommendations For Testing Managing And Treating Hepatitis C Virus Infection Ghany
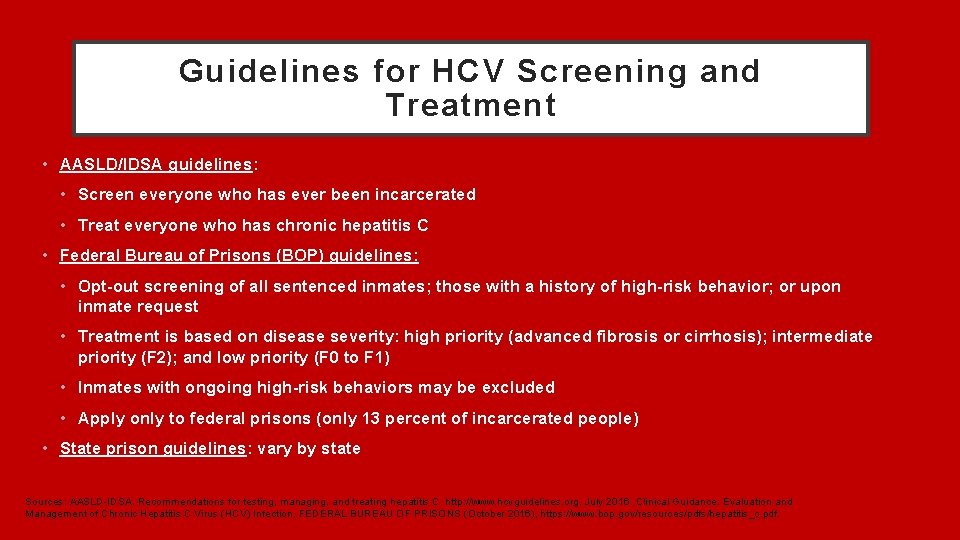



Hepatitis C Screening And Treatment In U S



Liverfoundation Org Wp Content Uploads 17 09 Hepatitis C Pdf
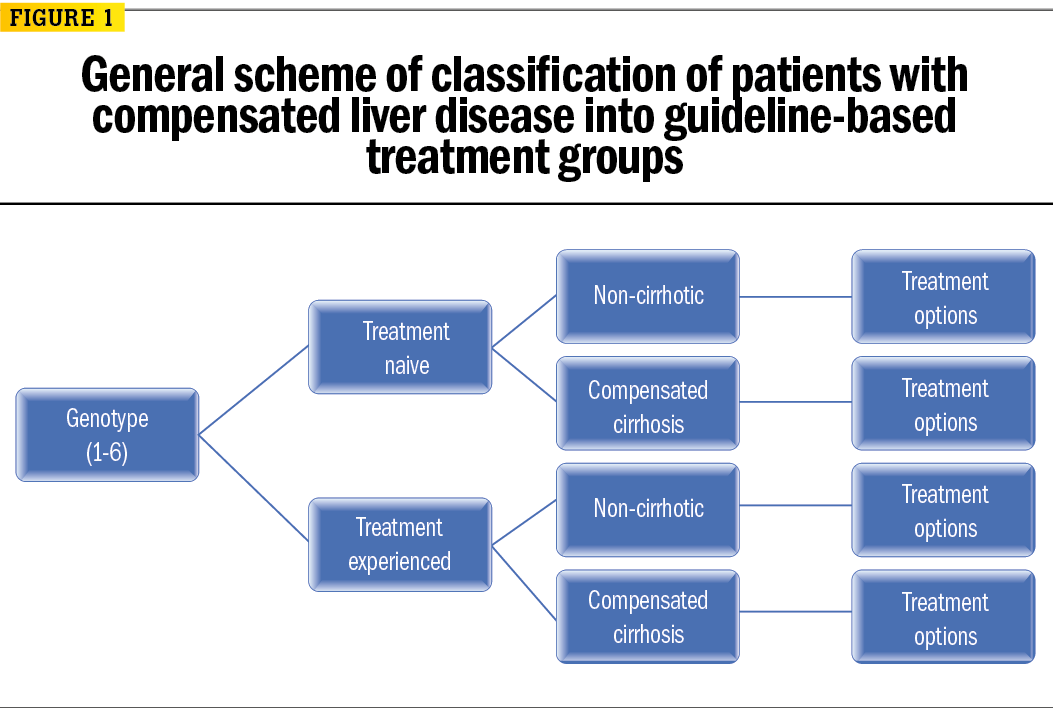



Advances In Hepatitis C Treatment




Hepatitis C Guidance 18 Update sld Idsa Recommendations For Testi
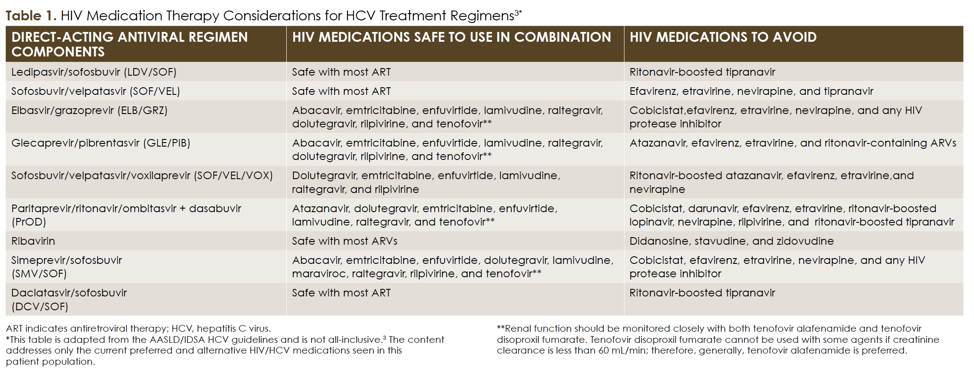



Treating Hepatitis C Virus And Hiv Coinfections In Intravenous Drug Users
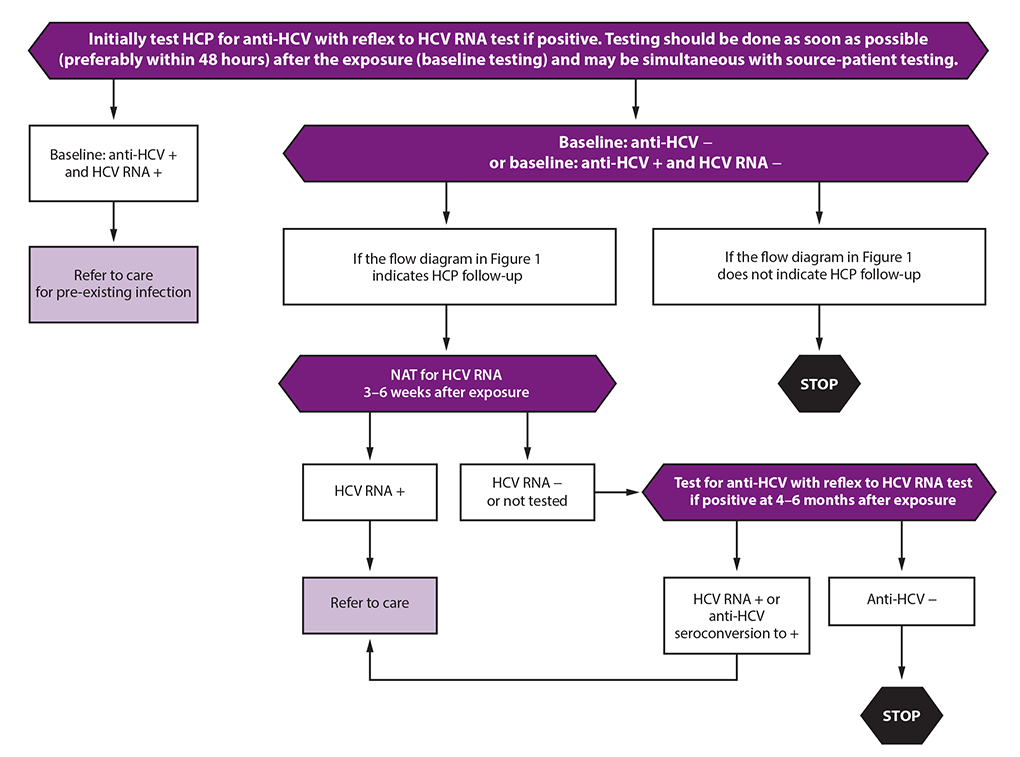



Testing And Clinical Management Of Health Care Personnel Potentially Exposed To Hepatitis C Virus Cdc Guidance United States Mmwr




Hepatitis C Guidance 18 Update sld Idsa Recommendations For Testi




Hepatitis C Guidance 18 Update sld Idsa Recommendations For Testi
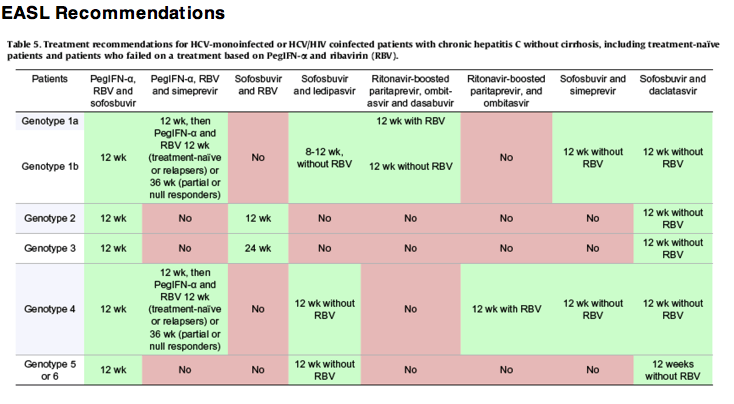



Genotype 3 Report Treatment History 3 Guidelines Easl Uk sld Idsa Ias Prevalence After Disease Progression




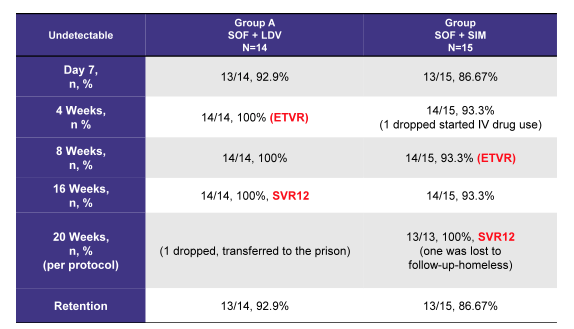
Hepatitis C In Prison Populations By Quantum Units Continuing Education Issuu
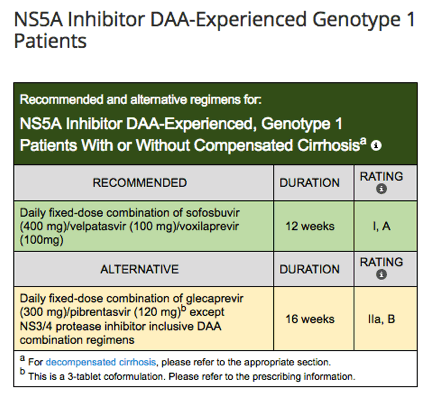



New Hcv sld Idsa Guidelines Update Sept 21 17 New Treatment Naive Treatment Experienced Guidance Genotype 1 Genotype 3
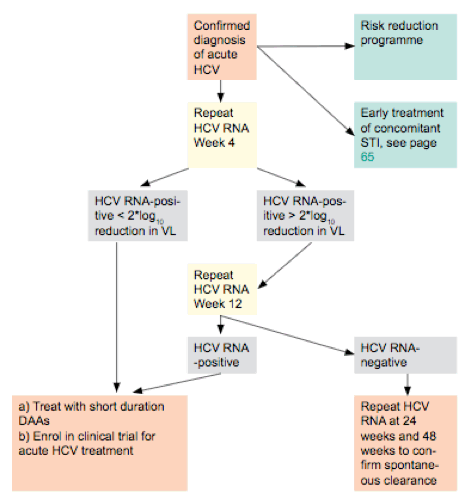



Summary From sld 17 For Hepatitis C Hcv Game Over




Hepatitis C Guidance sld Idsa Recommendations For Testing Managing And Treating Adults Infected With Hepatitis C Virus 15 Hepatology Wiley Online Library




Cdc Recommendations For Hepatitis C Screening Among Adults United States Mmwr




Hepatitis C Guidance 19 Update American Association For The Study Of Liver Diseases Infectious Diseases Society Of America Recommendations For Testing Managing And Treating Hepatitis C Virus Infection Ghany
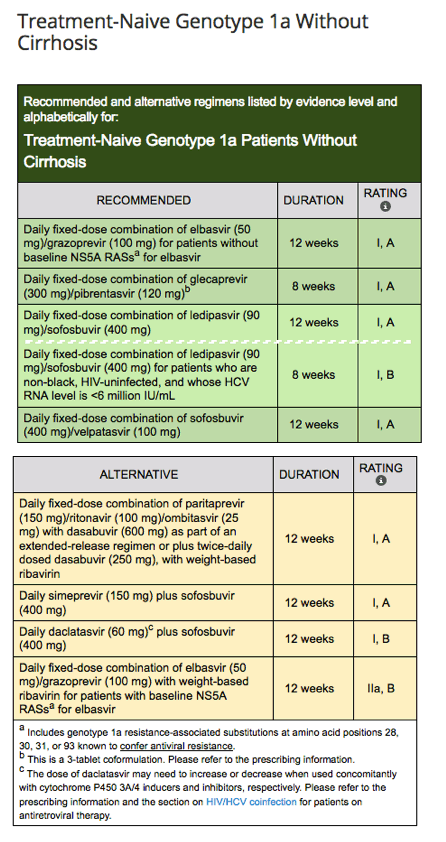



New Hcv sld Idsa Guidelines Update Sept 21 17 New Treatment Naive Treatment Experienced Guidance Genotype 1 Genotype 3
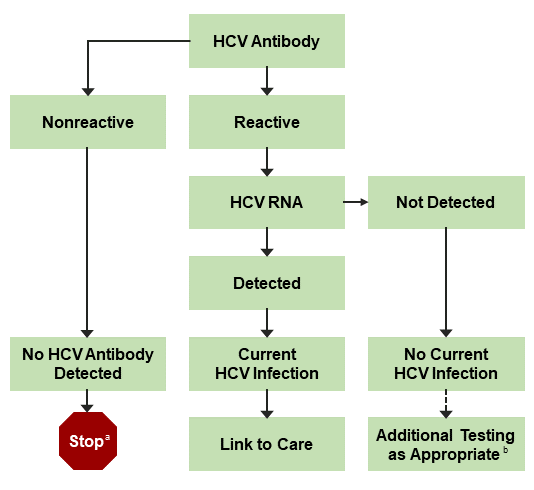



Hcv Testing And Linkage To Care Hcv Guidance




Hepatitis B Reactivation With Hepatitis C Treatment Bringing Some Clarity To The Black Box Gastroenterology




Pdf Easl Clinical Practice Guidelines Management Of Hepatitis C Virus Infection




Which Hcv Treatment Is Right For Me Positively Aware
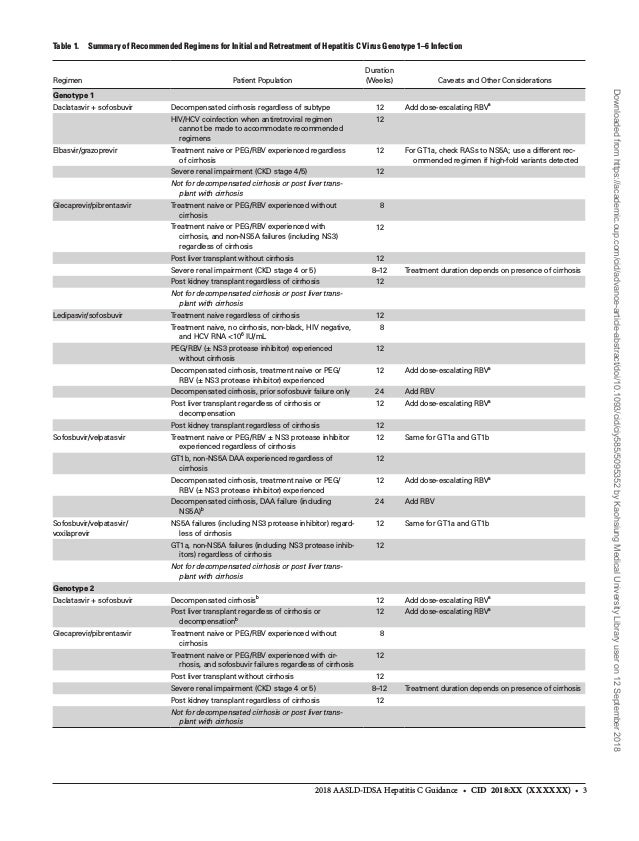



Hepatitis C Guidance 18 Update sld Idsa Recommendations For Testi
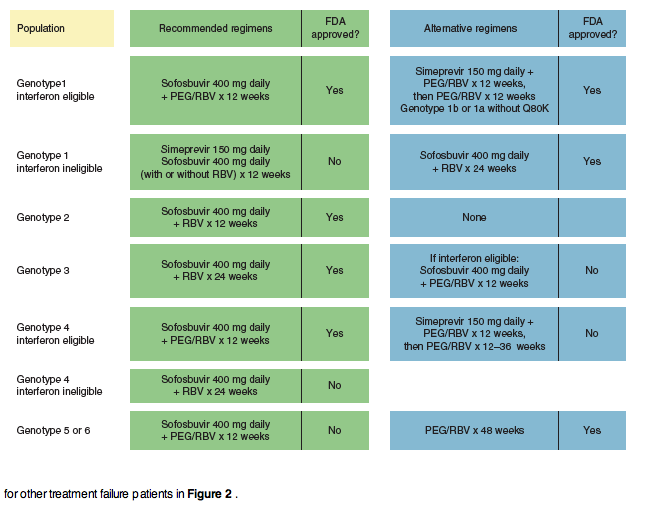



The Rapid Evolution Of Treatment Strategies For Hepatitis C Current sld Idsa Treatment Guidelines
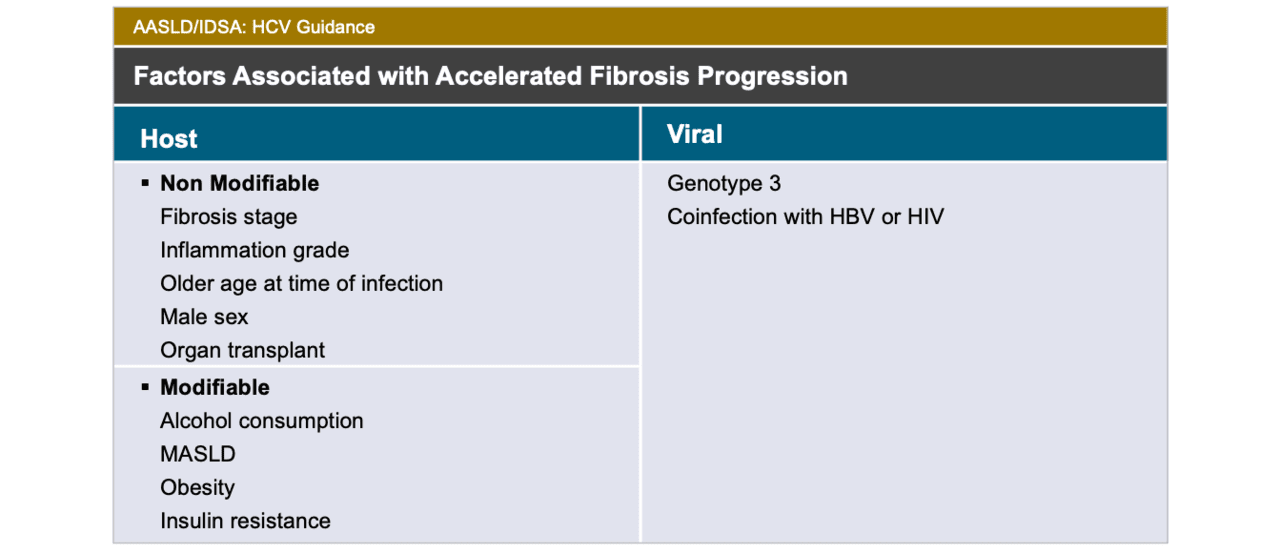



Core Concepts Making A Decision On When To Initiate Hcv Therapy Evaluation And Preparation For Hepatitis C Treatment Hepatitis C Online




sld Recommendations For Treatment Of Chronic Hepatitis C Download Table




Practice Guidelines sld




Where Are The Children In National Hepatitis C Policies A Global Review Of National Strategic Plans And Guidelines Sciencedirect
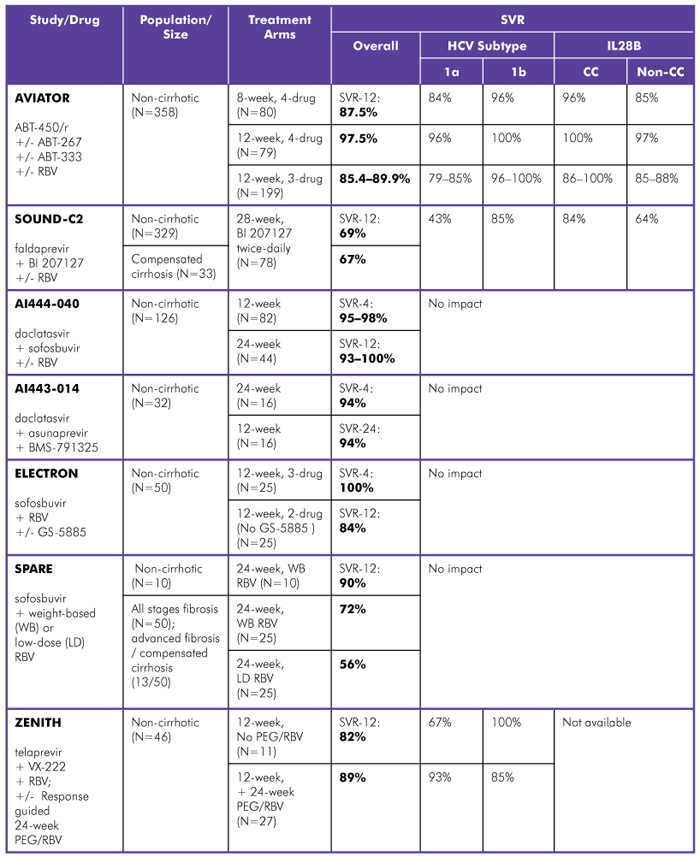



Hepatitis C Drug Development Update From sld Treatment Action Group
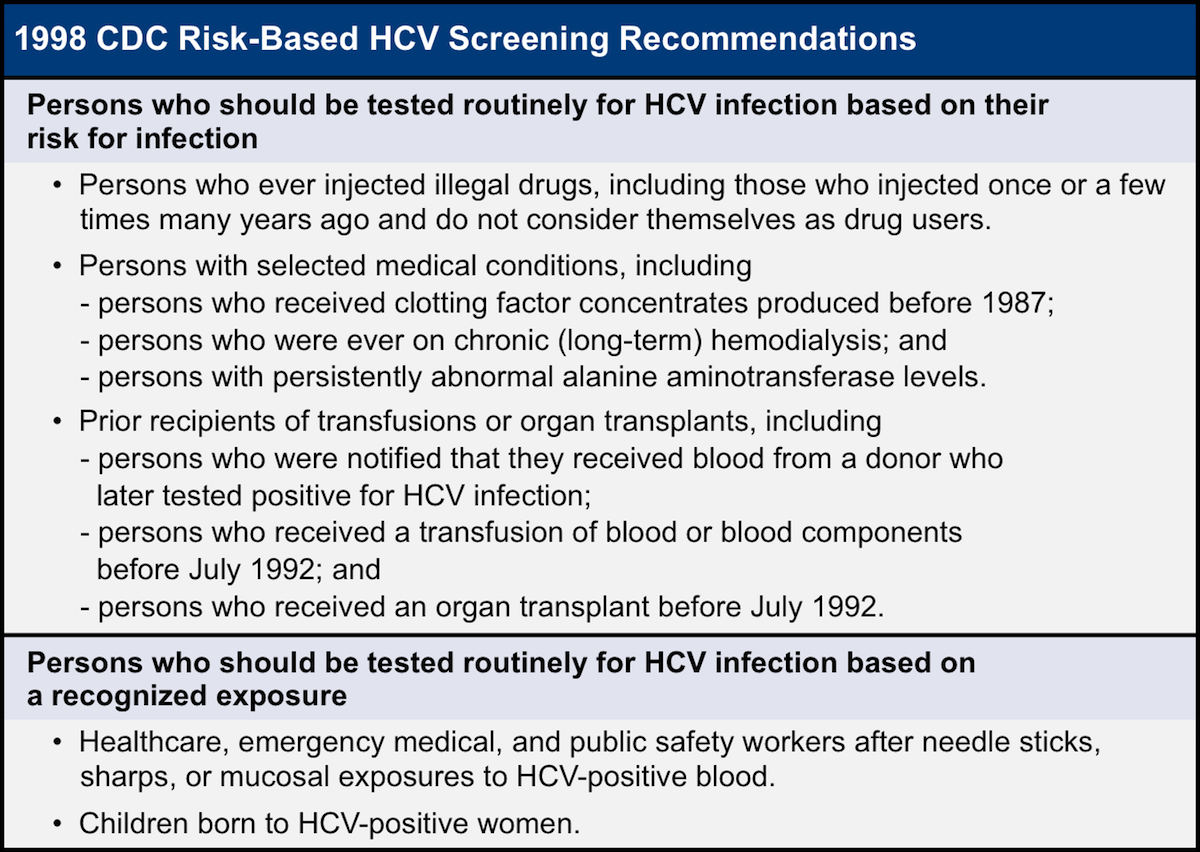



Recommendations For Hepatitis C Screening Core Concepts



0 件のコメント:
コメントを投稿